TEST REQUEST
Corporate studying toxicology for health and happiness of human.
Corporate studying toxicology for health and happiness of human.


Genetic toxicology exam observes a phenomenon which examination substance damages DNA or chromosome and causing morphological change or functional abnormality.
Genetic toxicology is a significant test item conducted at the screening level of development of new medicine or chemicals, and plays significant role for deciding the development.
Genogen Co.,Ltd conducts genetic toxicology test as condition for various guideline and conducts counseling for the test.
As for the types of genetic toxicology exam, there are various methods for verification of diverse toxicity, and the most general method is 3 batteries which are bacterial reverse mutation test, in vitro chromosomal aberration test, in vivo micronucleus test.
Reverse mutation is a test checking genetic toxicology by confirming the conversion of strains with deterred amino acid synthesis to amino acid synthesis strain by causing mutation from test chemical. The test method examines hereditary damage of DNA using more than 5 strains. It shows very close of correlation with the result of carcinogenicity test which is simple and rapid among other test methods. It is widely used to safety test of early screening level and medicine, food additive, agricultural pesticide, normal chemicals of new medicine development.
As the method to measure the structural abnormality of chromosome, In vitro chromosomal aberration test generally use Chinese Hamster Lung(CHL), Chinese Hamster Ovary(CHO) cell which is mammal cell.
Mouse Lymphoma TK assay test measure thymidine kinase gene mutation by test chemical, which generally use L5178Y tk +/- Mouse Lymphoma Cell.
In vivo Micronucleus test is used to detect abnormal chromosome caused in bone marrow cell or peripheral blood cell and measure the formation of micro-nucleus including the piece or whole chromosome.

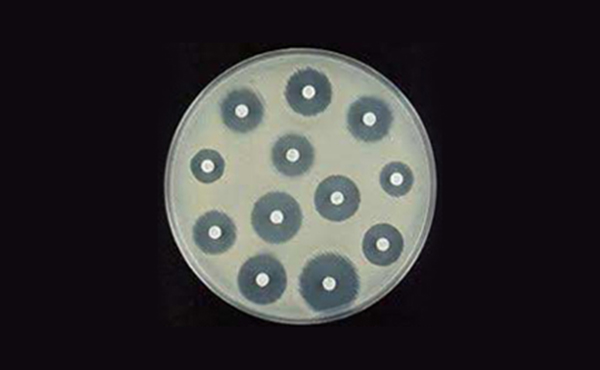
Due to the increase of interest toward hygiene, sanitary and stability based on improvement of living standard, many antibacterial products are in development. Antibacterial means resisting germs from growing, and antibacterial agent is used to kill germs by adding to raw material, ingredient and products. As for the verification test for antibacterial materials, we conduct MIC test, MBC test and Agar diffusion assay. MIC test is able to confirm the minimal concentration resisting growth of germ. and MBC test is able to confirm the sterilization concentration of 99.9%.


Cytotoxicity and genetic toxicology test for biological safety test of medical devices